|
Coronary artery disease is the most common cause of death in the United States, accounting for approximately 600,000 deaths annually. Of 6.0 million ED visits per year for chest pain, about 1.2 million people are diagnosed with myocardial infarction and another million with unstable angina. It has been estimated that the overall
cost of coronary artery disease exceeds 100 billion dollars annually in the U.S. There is also a significant cost in terms of malpractice claims, with missed myocardial infarction and acute coronary syndromes continuing to constitute a large percentage of both claims and costs. Mortality and morbidity continue to decrease with advances in therapy. There was a 54% reduction in age-adjusted mortality from myocardial infarction in the U.S. from 222/100,000 in 1963 to 101/100,000 in 1990.
Definitions
- Coronary artery disease (CAD) is a spectrum of disease that ranges clinically from asymptomatic or "silent" to one of the following clinical syndromes: stable angina, variant angina or acute coronary syndrome.
- Stable angina is episodic, exertional chest pain lasting approximately 5-15 min. EKG
changes occur less than 50% of the time. Cardiac enzymes are not elevated.
- Variant or Prinzmetal angina is uncommon and occurs primarily at rest without provocation, typically at the same time of the day. ST elevation can be seen on EKG.
- Acute coronary syndromes comprise the entire spectrum of disease from unstable angina through to myocardial infarction.
- Unstable angina is defined as new onset angina or angina that is increasing in frequency, intensity, duration, sensitivity to exercise or nitroglycerin (NTG) requirement. EKG changes occur in 50% or more of patients and cardiac troponin may be mildly elevated.
- Acute myocardial infarction (AMI) occurs when there is frank necrosis of myocytes. Chest pain is usually sustained >20 min. EKG changes occur in 50% or more of cases and cardiac serum markers are elevated. AMI is classically divided into transmural (Q-wave) or subendocardial (non Q-wave) MI, although there is significant overlap between these entities. A more important classification of AMI in the ED is to distinguish ST elevation MI (STEMI) from non-ST elevation MI (non-STEMI).
Pathophysiology
- In most cases, coronary artery disease is caused by atheromatous plaques in the lumina of the epicardial vessels. However, other sources of occlusion include thrombus formation associated with arterial dissections as well as thrombi from heart chambers and prosthetic valves. Inflammatory processes, such as those associated with
Kawasaki disease and systemic lupus erythematosis are uncommon causes of coronary artery disease.
- Stable angina is caused by chronic, fixed nonulcerating plaques. Angiography usually reveals 50-75% obstruction.
- Variant angina is primarily due to vasospasm. Angiography reveals normal coronariesin one-third of the cases and CAD in conjunction with vasospasm in the remaining two-thirds.
- Acute coronary syndromes share the common pathophysiology of a fissuring or "unstable" plaque (often with less than 50%occlusion) that becomes a nidus for the aggregation of platelets and fibrin. Vasospasm at the site of the fissured plaque also occurs.
- Mechanisms of cardiac ischemia and infarction independent of coronary artery obstruction include those from metabolic, hematologic and toxicologic conditions. Hypoxia, anemia, hypotension and carbon monoxide toxicity all tend to result in injury in a global cardiac distribution rather than in the distribution of a particular epicardial
artery, but may manifest first in territories of vessels with preexistent CAD.
Risk Factors
- Major risk factors for CAD include age, male gender, diabetes mellitus, chronic hypertension, family history of premature CAD, cigarette smoking, hyperlipidemia and lack of hormone replacement after menopause.
- Because these well known risk factors are based on lifelong risks in populations, they are less important in the ED than they are in the primary care setting. In the ED, the individual patient’s presenting symptoms (e.g., "crushing retrosternal chest pain versus sharp, intermittent pain") and the appearance of the 12-lead EKG (e.g., ST segment/T wave changes versus normal) overshadow any predictive value of the classic risk factors.
Diagnosis and Evaluation
History
- The classic presentation of symptomatic CAD is that of left-sided or retrosternal chest pain of a pressure-like nature. However, many variations exist including burning pain, pain akin to indigestion (approximately 20% of patients) and sharp, stabbing pain (5-20% of patients). Pain may radiate to the jaw, neck, back or down either upper extremity, corresponding to the C8-T5 dermatomes. It is important to note that lack of classic characteristics of pain cannot be used to rule out CAD as a cause for chest pain.
- The temporal pattern of pain and its relation to activity will help to classify the presentation into one of the clinical categories outlined above.
- Common associated symptoms include nausea and vomiting (especially with inferior
wall ischemia), diaphoresis, shortness-of-breath and lightheadedness.
- In diabetic and elderly patients, chest pain itself may be absent. In some of these cases an
"anginal equivalent" such as shortness-of -breath, lightheadedness or nausea may be present.
In the oldest subset of patients, CAD may present very nonspecifically with weakness, malaise or simply with the complications of an acute coronary event such as congestive heart failure (CHF), dysrhythmia or cerebrovascular accident.
- 5-15% of AMIs are completely asymptomatic or "silent". These occur mostly in elderly and diabetic patients.
Relationship of EKG leads to location of ischemia related epicardial vessel
| EKG Lead |
Location of
Ischemia/Infarction |
Epicardial Vessel |
| II, III, and AVF |
Inferior Wall |
Right Coronary Artery |
| V1, V2 |
Septal Wall
Posterior Wall* |
Left Anterior Descending Artery
Right Coronary Artery (in most cases) |
| I,AVL, V5 and V6 |
Lateral Wall |
Left Circumflex Artery |
| V1, V2,V3,V4,V5 |
Anterior Wall |
Left Anterior Descending Artery |
| Special Leads |
|
|
| V8, V9 |
Posterior Wall |
Right Coronary Artery |
| RV4 |
Right Ventricle |
Right Coronary Artery |
Note: In posterior wall ischemia/infarction, changes in leads V1 and V2 will be opposite
to those expected in other leads. Because ST segment changes in leads V1 and V2 can
represent both STEMI in the posterior wall as well as non-STEMI in the anterior and septal
locations, additional leads (e.g., V8 or V9) or an echocardiogram may be obtained to help
clarify the situation. The presence of a tall R wave in lead V1 is the equivalent of a Q wave
in the other infarct locations.
Physical Examination
- Physical examination is rarely diagnostic of symptomatic CAD. Although signs such
as the new onset of an S4 gallop and a paradoxical spilt of S2 have been described with
AMI, these are unreliable.
- The physical examination serves two other important roles:
- The exclusion of other life-threatening causes of chest pain such as pneumothorax
(asymmetrical resonance to percussion), aortic dissection (asymmetry of pulses, neurological signs) and pulmonary embolus (signs of right-sided heart strain)
- The identification of complications of myocardial ischemia and infarction such as
CHF (jugular venous distension, S3 gallop, rales, edema, hypotension), acute papillary muscle rupture and myocardial rupture (new onset murmur with signs of severe CHF) and pericarditis (friction rub).
EKG Findings
- The EKG remains the most important diagnostic tool in the evaluation of acute chest pain and guides the early management of patients with CAD. A 12-lead EKG should be obtained as soon as possible.
- There is a great degree of variability in the patterns of change on the 12-lead EKG during symptomatic CAD. In unstable angina and subendocardial MI, when a partial obstruction to flow in a coronary artery exists, T wave inversions and ST segment depressions are most commonly seen. ST elevations in the vascular territory of a particular epicardial artery (see above) is characteristic of transmural MI, when there is
complete obstruction.
- Because of the time-dependent nature of revascularization treatments for STEMI, the most important distinction that must be made in the initial evaluation of a patient with symptomatic CAD is that between those patients with significant ST elevation and those without.
- Since ST elevation may not be present on the initial EKG, it is important to obtain
frequent serial EKGs, especially when there is a dynamic nature to the patients presenting symptoms. Conversely, ST elevation on the initial EKG may completely resolve with medical therapy, again with major implications on therapy. For similar reasons, a previous EKG tracing of the patient prior to the acute episode may be extremely valuable in making emergency management decisions.
- Although variable, the typical progression of EKG changes in STEMI begin with the
brief (minutes) appearance of giant or "hyperacute" T waves, followed by ST elevations in the distribution of the occluded or "infarct-related" vessel. With time (typically several hours), there is the development of Q waves. T wave changes are variable.
- The presence of a left bundle branch block (LBBB) is extremely important in the evaluation of ACS. Its presence indicates a poor prognosis and the need for more aggressive management. If the LBBB is documented or presumed to be new in onset, the treatment of ACS follows similar lines as that of STEMI.
- The progression of nonSTEMI is even more variable than with ST elevation MI. Q
waves do not usually occur.
- The distribution of ischemia/infarction within the heart is important to predict likely
complications as well as to predict prognosis.
- The additional leads V8 and V9 may be placed when there is suspicion for acute
posterior wall MI, in which case ST segment elevation in these leads will be seen.
Suspicion for posterior wall MI exists when there are changes suggestive of ischemia in
leads V1 and V2.
- The additional lead RV4 may be placed when there is suspicion for acute right ventricular MI. Acute RV infarction is confirmed by the presence of ST segment elevation. Right ventricular MI may be suspected either on clinical grounds (e.g., because of an exaggerated hypotensive response to preload reduction therapy such as nitrates) or when ischemic changes are present in the inferior leads (II,III and avL).
- Changes of a dynamic nature (e.g., changes from previous EKG recordings on the same patient or changes over serial EKGs in the ED during the initial hours of the presentation) are more suggestive of ACS and predict a higher mortality and need for aggressive therapy.
- A normal EKG cannot be used to rule out the presence of ACS or AMI. However, in those patients with normal or minimally abnormal EKGs, the risk of mortality and complications is much lower.
Serum Cardiac Markers
- Cardiac markers have a role in the diagnosis as well as the risk stratification of patients
with ACS.
- At the present time, no perfect marker of ACS exists, although there ongoing advances in
technology and research continue to improve the utility of cardiac markers.
Table erum markers in acute coronary syndromes*
| Marker |
Time to
Elevation
(hours) |
Time to
Peak
(hours) |
Time to
Normalization
(days) |
| Myoglobin |
2 |
8 |
1 |
| CK (creatine phosphokinase) |
6-8 |
24-30 |
3-4 |
| CK-MB isoenzyme |
3-4 |
18-24 |
2 |
| Cardiac troponin I and T |
3-6 |
18-24 |
7-14 |
- The above figures are estimates. Characteristics of individual assays, as well as normal and diagnostic values, vary with technique and manufacturer. Laboratories will be able to provide specific information on marker assays used in individual institutions.
- Most institutions have a protocol for the serial measurement of cardiac markers in the
evaluation of possible ACS. Regardless of the protocol used, a knowledge of the benefits and limitations of each marker is important.
- Myoglobin, CK and cardiac troponin are all released into the blood after cardiac cell
injury and death. Table 2B.2 compares the temporal characteristics of these markers
in AMI.
- Myoglobin is elevated quickly and is very sensitive. However, its total lack of specificity limits its diagnostic value.
- CK-MB assays are generally less specific and less sensitive than cardiac troponin, although differences in technique account for significant variations in reported accuracy.
- To date, cardiac troponin I and T are the most cardiac specific markers and have been
adopted by many institutions in the U.S.
- Before the advent of cardiac troponin, the commonly accepted definition of AMI included an elevation in CK-MB. A new group of patients has been identified in whom there is a mild elevation of cardiac troponin without an increase in CK-MB, making the precise definition of AMI less clear. However, identification of this group of patients with ACS and "microinfarction" has proved important clinically, because they have poorer outcomes than patients with no marker elevations and warrant more aggressive management strategies.
- Although there is some degree of elevation of cardiac troponin in renal failure, it still appears to have prognostic value.
- In certain cases it may be helpful to combine markers because of their different temporal profiles. For example, the addition of CK to cardiac troponin may be helpful to detect early reinfarction in the week following an AMI when troponin values are still elevated from the initial event.
Other Laboratory Values
- When suspicion for ACS is high, additional laboratory investigations are appropriate and may include CBC, basic chemistry, urine toxicology and blood typing (when fibrinolytic or invasive procedures are contemplated).
Imaging Studies
- Emergent portable chest X-ray helps to rule out other important causes of chest pain such as aortic dissection and pneumothorax. Many institutions have protocols to ensure that a chest X-ray is reviewed prior to the administration of fibrinolytic therapy so that signs suggestive of aortic dissection are not overlooked. Chest X-ray is also important to detect cardiomegaly and other signs of congestive heart failure
that may complicate or coexist with ACS.
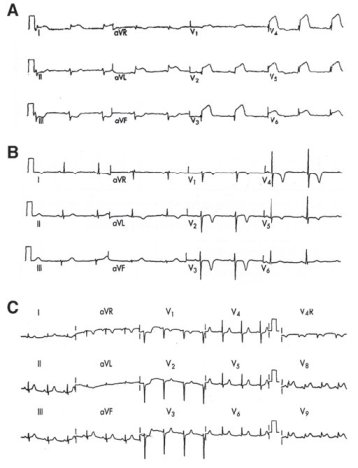
Figure 2B.2. Important EKG findings in acute coronary syndromes. A) Acute anterior MI showing ST elevations in the precordial leads, with reciprocal ST depression in the inferior leads (reciprocal changes increase diagnostic certainty of AMI and also indicate a graver prognosis). B) Deep, symmetrically inverted T waves in the anterior leads in setting of cardiac symptoms are strongly suggestive of proximal left anterior descending obstruction and carry a poor prognosis without prompt therapeutic intervention. Although emergent cardiology referral and admission to the CCU are appropriate, this patient does not meet criteria for the administration of fibrinolytics. C) A 15-lead EKG performed in a patient with ST segment depressions in leads V1 and V2. In this case, the finding of ST elevation in leads V8 and V9 confirms that the changes in V1 and V2 actually represent an acute posterior infarction rather than only septal ischemia—this patient now meets criteria for revascularization therapy with fibrinolytics or percutaneous coronary intervention (PCI)
- Emergent echocardiography has an important role in cases where the diagnosis of ACS or MI is still in question after the initial assessment and EKG.
Echocardiography gives a great deal of information that may guide management, including an assessment of the global functioning if the heart (ejection fraction), assessment of regional wall motion and the function of the cardiac valves.
- In the setting of ACS with cardiogenic shock, echocardiography is also useful to rule out mechanical complications such as papillary muscle, septal and free-wall rupture that mandate emergent surgical consultation. At present, bedside ultrasound by EPs is limited to the detection of pericardial effusion, which may by itself be helpful in some cases.
ED Management
- As with every patient in the ED, management begins with the primary survey and emergent resuscitation as required.
- All patients being evaluated for ACS must be placed on a cardiac monitor with a pulse oximeter. IV access should be obtained in all patients. Intubation and resuscitation equipment, including an external pacer and defibrillator, must be checked and ready for use at the bedside.
- Treatment of symptomatic CAD is tailored in each case to the severity of the presenting syndrome. All patients with presumed ACS receive the most basic treatments, with the most aggressive therapies reserved for massive STEMIs. In general:
- The treatment of STEMI or ACS with presumed new LBBB focuses on immediate revascularization therapy with fibrinolytics, percutaneous coronary intervention (PCI) or surgery.
- The treatment of non-STEMI ACS focuses on reduction in myocardial necrosis and the prevention and treatment of complications.
- The treatment of patients with stable angina or chest pain of uncertain etiology focuses on the elimination of ACS from the differential diagnosis and risk stratification for future coronary events.
Oxygen
- Oxygen should be given to all patients. It may be provided as 2-4 L/min by nasal cannula or by face mask in the presence of hypoxia.
Aspirin (ASA)
Aspirin (ASA) decreases platelet aggregation by inhibition of cyclooxygenase, which results in decreased production of thromboxane A2.
- ASA is the single most effective medication in treatment of CAD and reduces mortality by over 20% in ACS. All patients should receive ASA except those with true allergies or active hemorrhage. Concurrent antacid use can decrease GI upset.
- The dose in ACS is 160-325 mg PO (lesser doses may be acceptable for chronic therapy, but not for ACS)
- Ticlopidine (Ticlid) and clopidogrel (plavix) are alternative agents for patients with severe (anaphylactoid) reactions to ASA. These agents have a slower onset of action, more adverse reactions and are more expensive than ASA. Their role in the ED is very limited at this point, although an additive benefit of clopidogrel to ASA therapy was identified in one recent trial.
Nitrates
Nitrates cause vasodilatation of coronary arteries, relieve vasospasm, and decrease preload and afterload, which in turn decreases myocardial oxygen demand.
- Evidence for their benefit in ACS is indirect at best. Nonetheless, nitrates have an important role in relieving symptoms and improving hemodynamics in ACS.
- Patients with systolic BP>90 Hg mm and ongoing chest pain should be treated with
nitroglycerin (NTG).
- Caution should be exercised when using the sublingual dose (0.4 mcg SL Q3-5 min) because it can cause profound hypotension, especially in right ventricular infarction.
- Nitrates are contraindicated when sildenafil (Viagra®) has been used in the preceding
48 h.
- If pain continues after the initial 3 doses of sublingual NTG, it may be administered by IV infusion, starting at 10 mcg/min and quickly titrated upward to resolution of pain while maintaining systolic BP >90.
- If symptoms are mild, NTG may be given by paste applied to the chest wall. Doses range from 1/2-2 inches of topical paste.
Morphine
- Morphine is a potent analgesic and sedative. Evidence for its effectiveness in AMI is indirect, but it is very effective at decreasing patient anxiety and fear. Similar to nitrates, caution is advised when systolic BP is low. Even small doses (e.g., 2 mg) may be beneficial.
Beta-Blockers
Beta-blockers exert their effects by decreasing afterload, contractility, overall myocardial oxygen demand and myocardial irritability.
- Studies have shown that early use of ß-blockers in AMI reduces infarct size and reduce mortality significantly.
- Beta-blockers should be administered to all patients without contraindications, which include cardiogenic shock, hypotension (systolic BP less than 90), reactive airway disease (asthma and COPD), allergy, advanced (2nd or 3rd degree) AV blocks and bradycardia
- Atenolol and metoprolol are B1 selective IV agents and are preferred. Metoprolol can be given in 5mg increments IV q 5min, to a usual total dose of 15 mg. Esmolol, which is an IV titratable agent with a half-life of under 10 min, can be given in cases where reversibility is desired (e.g., in the presence of relative or uncertain
contraindications)
Unfractionated Heparin (UFH) and Low Molecular Weight Heparins (LMWH)
UFH and LMWH are antithrombin agents. Their effectiveness in ACS and AMI is supported by some studies but is controversial. In addition, the benefits of antithrombin agents appear to disappear once they are discontinued. UFH and LMWH are also not without risk, with a very significant incidence of life-threatening bleeding complications. Because of the potential for hemorrhage, risk/benefit ratios for the use of these agents must be considered in each individual case.
- Although some experts recommend their use in all cases of ACS in the absence of a specific contraindication, they may be more appropriately limited to a subset of patients at highest risk for mortality and complications (e.g., those with significant EKG changes) as a "bridge" to more definitive revascularization therapy.
- Unfractionated heparin (UFH) is administered according to ideal body weight with the usual dose of 60 µ/kg bolus followed by an infusion of 12 µ/kg/h (maximum 4,000 u bolus and 1,000 µ/h infusion). PTT must be monitored regularly starting at 4-6 h after the initiating of the infusion) with a goal of maintaining PTT at 2x the
upper limit of normal.
- Low molecular weight heparins (LMWH) have been used in many trials. They are as effective as UFH and have several advantages, including ease of administration (subcutaneous injections versus IV infusion), more consistent therapeutic effects, and a lack of need for routine therapeutic monitoring. It is also possible to use LMWH in patients for whom PCI is anticipated, although heparin is still preferred by some in this setting because of its easier titratability. A commonly used LMWH is enoxaparin, with a dose of 1mg/kg SQ q12h.
Glycoprotein IIb/IIIa Receptor Inhibitors
Glycoprotein IIb/IIIa receptor inhibitors (abciximab, eptifibatide, tirofiban) are very potent anti-platelet agents that block the final common pathway of platelet aggregation (the formation of fibrinogen bridges between platelets). They are administered as IV infusions in ACS.
Primary Percutaneous Coronary Intervention (PCI)
PCI is becoming increasingly popular in U.S. centers and in experienced hands provides outcomes superior to fibrinolytic therapy for STEMI, particularly when coronary stenting is employed.
- Primary PCI has an advantage over fibrinolytic therapy when cardiogenic shock complicates AMI. It may be the only option for revascularization when there are absolute contraindications to fibrinolytic therapy. It also may be preferred in patients >75 yr of age who have higher rates of complications with fibrinolytics.
- The most important factor in outcome remains time to revascularization. Therefore, any benefit of PCI may be lost if there is significant delay in bringing the patient to the catheterization laboratory. A delay of more than 90 min is not likely warranted, and if such a delay is anticipated, administration of fibrinolytics should proceed. A reasonable goal is to perform revascularization by interventional or pharmacological means within 60 min or less from the time that the patient arrives in the ED.
- In some cases, PCI may be considered after fibrinolytics, especially when there is a
poor response to therapy.
Complications
The two complications of ACS responsible for the majority of deaths are dysrhythmias and congestive heart failure/cardiogenic shock.
Arrhythmias
- Arrhythmias are very common after an ischemic event. The incidence of premature ventricular contractions (PVCs) is virtually 100% and sinus tachycardia has an incidence of 40-60%. Ominous rhythms such as ventricular tachycardia and/or ventricular fibrillation have an incidence of 5-10%.
- The risk of a life-threatening dysrhythmia complicating ACS generally increases with infarct size and is greatest in infarcts of the left anterior descending artery (anterior) distribution.
- Anterior wall MIs are more likely to be complicated by severe bradydysrhythmias (e.g., Mobitz II and third degree AV block) due to injury of the conducting system.
- These rhythms may not respond to atropine and preparations for external and/or invasive pacing should be made immediately.
- Inferior wall MIs are often complicated by less severe bradydysrhythmias (e.g., first degree AV block or Wenkebach patterns) due to an increase in vagal tone. These are usually transient and responsive to IV atropine.
- Tachydysrhythmias increase myocardial oxygen demand and should be treated according to ACLS protocols.
- It should be noted that the presence of low-grade ectopy in ACS, such as intermittent
premature ventricular contractions (PVCs), is not routinely treated with
antidysrhythmic agents as was once common practice.
Left Ventricular Failure/ Cardiogenic Shock
- Left ventricular failure and cardiogenic shock are more common after anterior wall AMI because of the typically larger infarct size.
- Vasoactive catecholamines such as dopamine, dobutamine, epinephrine and norepinephrine all have a role in management, but all increase cardiac irritability and oxygen consumption, making them less than optimal treatment modalities. An arterial line and Swan-Ganz catheter should be used to guide therapy when these agents are used.
- Intra-aortic balloon pump (IABP) is another important tool that may be used in cases of refractory shock, especially as a bridge to revascularization or transplantation surgery.
- Ventricular septal defect and papillary muscle rupture should be sought as causes of shock and sudden decompensation; 50% of all cases occur in the first 5 days of an MI and 90% in the first 14 days. If these are suspected, emergent cardiovascular disorders surgical consultation is indicated.
Disposition
- All patients with suspicion for ACS should be admitted to telemetry or a chest pain unit for observation.
- For those patients in whom AMI is ruled out by serial cardiac markers and EKGs (usually over a time period of 8-12 h), the diagnosis of unstable angina is still a possibility. In the case of a patient with significant risk factors for CAD or typical symptoms, further risk stratification can occur with provocative testing, such as stress
echocardiography or nuclear medicine studies, on an inpatient basis. For those patients with atypical presentations of chest pain with few or no risk factors for CAD that are felt to have a very low likelihood of occlusive disease, timely outpatient follow-up (2-3 days) with a primary care provider is appropriate.
- Patients with a confirmed diagnosis of AMI should be admitted to a cardiac or intensive care unit (CCU or ICU).
|